Adverse events
The collection of adverse events (AEs) and serious adverse events (SAEs) forms an important part of BSRBR-RA.
What is an adverse event (AE)?
“Is any untoward medical occurrence in a patient being administered a pharmaceutical product, which does not necessarily have a causal relationship with the product.”
Examples of adverse events include, but are not restricted to;
- Any new diagnosis
- Worsening of a pre-existing condition
- Clinically significant laboratory results
- Any event that has been considered to be of sufficient importance to document in hospital case notes. These may include nausea, weight gain, headaches etc.
While adverse events can be directly attributed to a drug the patient is receiving, they do not have to be. On the other hand, any side effects should be recorded even if they are known to be caused by a drug the patient is receiving.
What is a serious adverse event (SAE)?
A serious adverse event or reaction is an untoward medical occurrence that is considered to represent a significant hazard to the patient. This definition is derived from regulatory authorities, including the European Medicines Agency (EMA) and the US Food and Drug Administration (FDA), and includes events that result in:
- Death
- Life-threatening (immediately)
- Overnight hospitalisation (initial or prolonged)
- Requires an intravenous antibiotic/anti-viral/fungal drug
- Significant loss of function or disability
- Congenital malformation/birth defect
- Other medically important event*
- Pregnancy
* Other medically important event is one that, if left untreated, could lead to any of the previously listed SAE categories. Examples include malignancy, allergic bronchospasm requiring intensive treatment in A&E or at home.
Recording AEs/SAEs
AEs and SAEs can be recorded when a patient’s follow up is due for completion. There is no requirement to inform BSRBR-RA as they occur.
The most essential pieces of information when recording an event are:
- Date of event*
- Diagnosis
- Biologic/targeted therapy details at time of event (these should be recorded in the Biologic Targeted Therapy section of the follow up record)
- Was the event serious & why? (SAE criteria e.g. required IV antibiotics)
- Is the event related to the biologic/targeted therapy?
* If unavailable, please estimate these dates and select the ‘This is an estimated date’ box. If only year is known use 15/JUN and if only month/year is known use 15 for the day. Please do the same for unknown admission/discharge dates.
Where this information is not available, particularly in the case of a death, it is really important that you report the event as a SAE in the current follow up record and add that you have no further details around the event in the Detailed Event Description box, so that we don’t request further information from you.
Deaths
All deaths must be recorded in the adverse event section of a follow-up record. Please include all information that you have available in the adverse event section, particularly the date and cause of death. If there is any information not available, please make a note of this in the "detailed description of the event" box. Please also complete the follow-up record as fully as possible, including the biologic targeted therapy section. If a follow-up record is not available for you to complete, please contact the team, who will assist with this.
Elective procedures
Elective procedures (e.g. joint replacements) that include overnight hospitalisation are not automatically considered as a serious event unless the hospital stay is prolonged due to complications (e.g. infection). However we would still like to know about planned surgeries, and in particular why they were performed, as it will be the indication for the surgery that is an event rather than the procedure itself.
Events of special interest (ESIs)
Some adverse events are classed as an Event of Special Interest (ESI). The way we capture this information has changed. For these events, we will use the query system in the database if we need further information from you, with the exception of serious infections (excluding TB), where you can complete the serious infection ESI form when the event is entered in the follow-up record.
How to enter an ESI form
1. Once you have recorded the serious infection in the adverse events section of the database, select Add New ESI category:
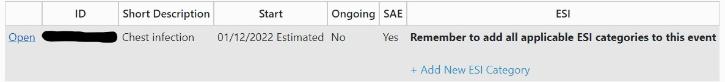
2. Then select Serious infection and click insert:
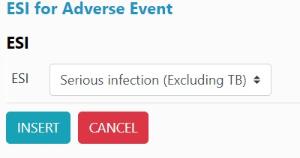
3. Click add form:

4. Please complete the questions and click insert form once completed:
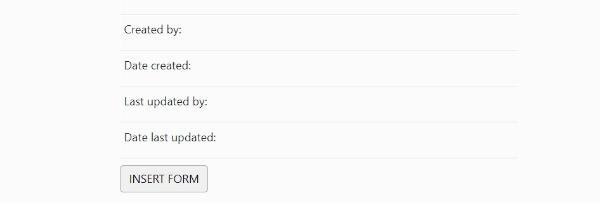
We may contact you to ask for additional information regarding an event. If you have no further details, please let us know, in order to prevent repeated requests.
How to complete an ESI form when the FUP edit window has closed
Once the edit window for a Follow up has closed, you will need to follow these steps to complete an ESI form that has been attached to a SAE (usually by a member of the BSRBR-RA team):
1. On the patient summary page, in the Menu on the left side of the page, you will see ‘Missing ESI Form’ in red. Click on this.
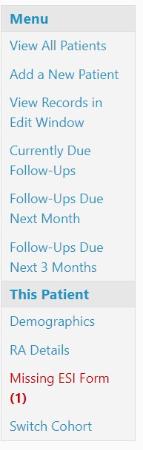
2. Click on the blue ‘Upload’ button to the right of the SAE you are completing the ESI form for:
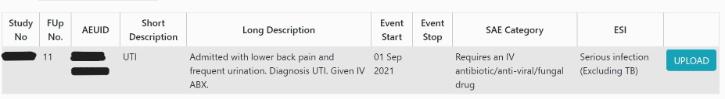
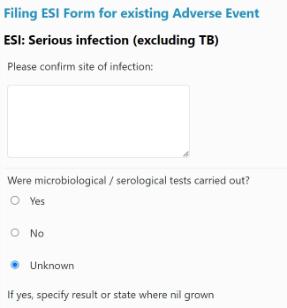
3. Scroll down the page until you come to the ESI questions for completion. Complete these questions.
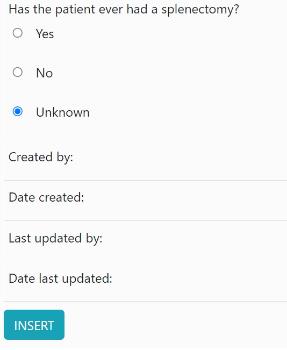
4. Once all the questions have been completed, press the ‘Insert’ button at the bottom of the page:
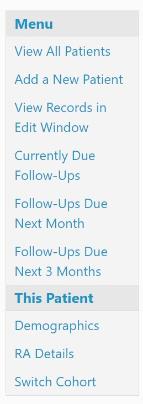
5. Once this has been done, the completed ESI form has been uploaded and ‘Missing ESI form’ will disappear from the menu on the patient summary page:
Yellow Card Scheme
In addition to reporting all suspected side effects and adverse drug reactions on the BSRBR-RA database, please remember to also report these directly to the MHRA using the Yellow Card Scheme.
Help with adverse events and ESIs
If you have any queries related to adverse events or ESIs, our pharmacovigilance team will be happy help you, either via the online querying system in the database, or by emailing BSGPharmacovigilance@manchester.ac.uk.
More information can also be found via our database training and tutorial pages, including details on how to request bespoke training regarding all aspects of AE/SAE recording.
