Database FAQ
Find answers to frequently asked questions on this page.
Use the links below to jump to the answer you're looking for.
- Q1: I've forgotten my database username or password - what should I do?
- Q2: My database account has been locked - what should I do?
- Q3: What do I do if a patient did not attend (DNA) their most recent follow-up visit?
- Q4: What do I do if a patient has not attended for a longer period of time, or if they have been discharged?
- Q5: What is the 14-day edit window and how do I close it?
- Q6: How do I enter drug doses?
- Q7: How do I switch a current participant to a new cohort?
- Q8: How do I indicate a dose/frequency change for a biologic or conventional therapy?
- Q9: How do I report an Adverse Event (AE), or a Serious Adverse Event (SAE) such as death? / How do I add an Event of Special Interest form to the database?
- Q10: Is there training available?
Q1: I've forgotten my database username or password - what should I do?
If you've forgotten your username, and you have tried using your email address, please get in touch. If you provide us with your email address we will be able to find your account and tell you your username.
If you've forgotten your password, you can use the Forgotten Password page on our database. Enter your username and a new temporary password will be emailed to you. If the email does not arrive within a couple of minutes, please check your spam/junk folder. We recommend that you immediately change the temporary password to something more memorable.
Q2: My database account has been locked - what should I do?
For security purposes, we automatically lock all database accounts that have not been used within the last six months. If you need your account to be unlocked, please contact us via email.
Q3: What do I do if a patient did not attend (DNA) their most recent follow-up visit?
As an observational study, we only require information that is in the patient notes. If there is information available from the notes applicable to the time period covered by the follow up, please enter this into the database.
If there is no information available at all, please let us know using the Feedback section so we can update the follow-up status to Missed / Data cannot be recorded. The follow up will be removed from your list of overdue follow-ups.
Q4: What do I do if a patient has not attended for a longer period of time, or if they have been discharged?
Please let us know if a patient has not attended for at least 2 years, or if they have been discharged, via the feedback section. We can then update the status and remove the patient from your list of patients under active follow up.
Q5: What is the 14-day edit window and how do I close it?
The 14-day edit window is the period of time that you are allowed to add and change data within a registration or a follow-up. Once this period ends, you will be able to view the data that has been entered, but you will not be able to change it. If you have noticed a mistake and the edit window is closed, please contact us and we will be able to help.
The 14-day edit window begins when you add a new patient or open a follow-up for the first time, and ends on day 14 unless it is manually closed. Once closed, it is transferred to an inbox monitored daily by the BSRBR-RA admin staff, who will check the information entered in the follow-up and raise any data queries if necessary. See our Avoiding Data Queries page for more information on how to make sure you incur as few queries as possible!
We recommend that you close the edit window manually when all the information has been entered. Manually closing the edit window means that the baseline/follow-up will be checked sooner, usually within one working day of closing it and therefore queries can be raised more time efficiently.

Q6: How do I enter drug doses?
We only require drug doses for Rituximab infusions - for the following drugs, we need all dose dates:
- MabThera (Rituximab)
- Rixathon (Rituximab biosimilar)
- Ruxience (Rituximab biosimilar)
- Truxima (Rituximab biosimilar)
To enter these dates, on the Biologic Targeted Therapy section, click the edit button -
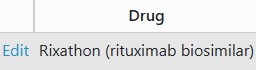
Add each dose date, dose and batch (if known) and click save -
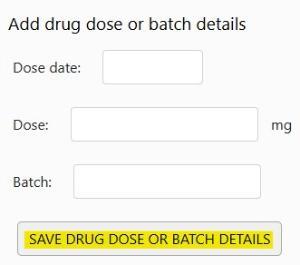
When you have entered all dose dates for the follow up, click on Save Page -

Drug doses are commonly missed and queried – if this information is not available, please let us know by leaving a note in the Feedback/comments section to avoid a query being raised.
Q7: How do I switch a current participant to a new cohort?
If a patient is already registered with us and has started a new therapy, they may be eligible to be re-registered. We need the following:
- Start date/dose/frequency of the new therapy (NB The therapy must be on the current eligibility checker).
- A DAS28 from around the time the patient started the new therapy (if available).
- A new consent form.
If all this information is available, search under the current ID and when you are on the Patient Summary page, click on Switch Cohort

You will be asked to complete the new therapy information. To complete the cohort switch request, click on -

We will then contact you about completing the process.
For more information on re-registering a patient, visit the Registration section of the website.
Q8: How do I indicate a dose/frequency change for a biologic or conventional therapy?
To represent a dose/frequency change on the BSRBR-RA database, please enter a stop date for the current course and enter a new course. The stop reason for the original course can be set as Other, with the text "Dose change" or “Frequency change” entered in the details box, as shown below:
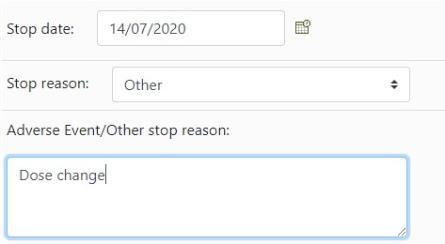
Once you have entered the stop details, save the page, click the Add New button and enter the details of the new dosage/frequency. Save this page and you should see something similar to the image below:
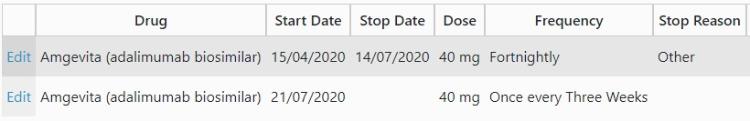
Q9: How do I report an Adverse Event (AE), or a Serious Adverse Event (SAE) such as death? / How do I add an Event of Special Interest form to the database?
For all of our information on Adverse Events and Serious Adverse Events, please see the following document on our database tutorials page:
Q10: Is there training available?
We run regular training sessions aimed at different audiences. Our current sessions are:
- Overview and database demo – aimed towards NHS professionals who are new to BSRBR-RA.
- Refresher – aimed towards NHS professionals who would like a refresher demo on how to use the database.
- Adverse events – aimed towards NHS professionals who complete follow ups and would like further information on what information we need specifically with regards to adverse events.
This blog post provides a little more information about the sessions. Look out for future dates in our regular monthly news updates.
